【论著】| AGPAT5在肝癌中的功能与机制研究
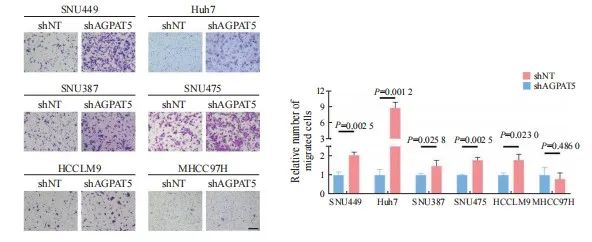
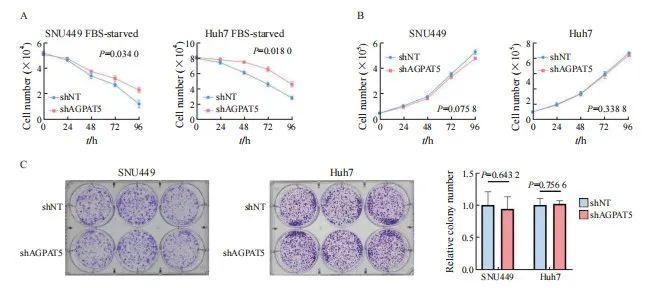
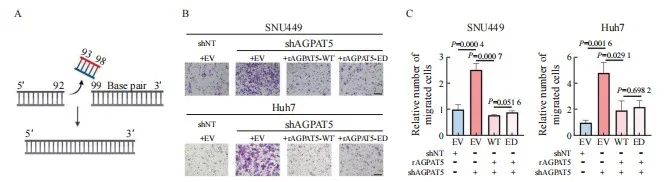
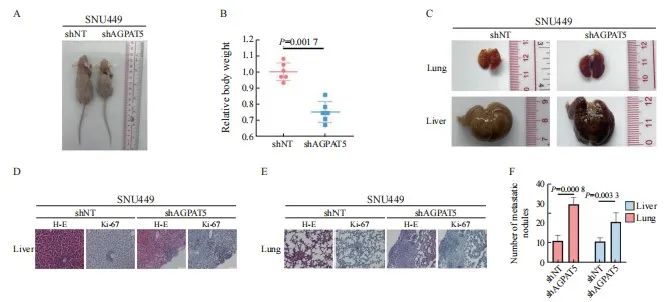
引用本文 陈奕君,刘雨航,段海波,等. AGPAT5在肝癌中的功能与机制研究[J]. 中国癌症杂志, 2024, 34 (9): 838-847. 基金项目:国家自然科学基金面上项目(82272714)。 第一作者:陈奕君,硕士。 通信作者:王雄军,教授、博士研究生导师。 第一作者简介 陈奕君,广州大学硕士研究生,生物化学与分子生物学专业,研究方向为肝癌。 通信作者简介 王雄军,广州大学教授,博士生/硕士生导师,入选2020年国家重大人才项目(青年长江学者)和广东省自然科学基金杰出青年项目。目前任中国抗癌协会肿瘤标志专业委员会青年委员、中国老年保健协会肝癌综合治疗专业委员会委员及广东省病理生理学会肿瘤专业委员会委员。长期从事消化道及泌尿系统肿瘤的代谢重塑与耐药,代谢产物调控的信号转导等方面的研究,在肿瘤代谢领域取得突破性的研究成果。目前,在该领域以通信作者或第一作者身份在 Nature、Cell Research、Hepatology、Molecular Cell、Journal of Clinical Investigation、Nature Communications、EMBO Journal、Advanced Science等权威杂志发表论文30余篇。作为项目负责人承担国家自然科学基金面上项目、国家重大研究计划培育项目,科技部重点研发子课题及广东省科技领域重点研发子课题。 作者解读 AGPAT5在肝癌中的功能与机制研究 陈奕君,刘雨航,段海波,王雄军 广州大学精准编辑与健康研究中心,广东 广州 510405 [摘要] 背景与目的:肿瘤的发生、发展过程中会发生代谢重编程,1-酰基甘油-3-磷酸O-酰基转移酶(1-acylglycerol-3-phosphate O-acyltransferase,AGPAT)作为三酰甘油(triacylglycerol,TAG)从头合成的关键酶,与肿瘤的进展密切相关。但目前作为亚型之一的AGPAT5在癌症中的研究还十分有限,本研究深入剖析AGPAT5在肝癌发生、发展中发挥的作用及潜在的分子机制,旨在为肝癌诊断和治疗策略提供新思路。 方法:利用慢病毒感染将多种肝癌细胞系中的 AGPAT5敲减,并通过锥虫蓝计数、划痕、transwell及平板克隆等实验在体外检测AGPAT5对肝癌细胞增殖、迁移及抗失巢凋亡能力的影响。通过回复野生型或酶活性缺失型的AGPAT5,探究其作为代谢酶是否发挥经典代谢作用调控肝癌细胞迁移。构建BALB/c裸鼠尾静脉注射移植瘤模型,从体内层面验证体外的细胞表型。采用免疫沉淀质谱联用(immunoprecipitation mass spectrum, IP-MS)鉴定出与AGPAT5相互作用的蛋白,并进行免疫共沉淀(co-immunoprecipitation,coIP)验证。蛋白质翻译后通过修饰鉴定分析AGPAT5潜在的修饰位点,通过体外实验探究点突变前后对肝癌细胞迁移的影响。通过coIP探究该位点突变前后AGPAT5与相互作用蛋白结合的情况。通过敲低相互作用蛋白确定其在细胞表型中的作用。通过回复实验验证AGPAT5是否通过相互作用蛋白发挥作用。检测野生型肝癌细胞系中的AGPAT5和相互作用蛋白的表达水平,检验两者之间是否具有相关性。 结果:肝癌细胞敲减 AGPAT5后会更加耐受无血清饥饿,并促进细胞迁移,但不会影响细胞增殖和失巢凋亡。而酶活性缺失并不影响AGPAT5对肝癌细胞迁移的抑制。敲减 AGPAT5可促进肝癌细胞在裸鼠体内的肺转移和肝转移。AGPAT5可以与原纤维蛋白(fibrillarin,FBL)相互作用,并在无血清饥饿刺激下加强两者的结合。遏制FBL的表达会抑制肝癌细胞迁移,且效果与过表达AGPAT5相似。抑制FBL的表达可削弱敲低 AGPAT5对肝癌细胞迁移的促进作用。在已检测的肝癌细胞系中,AGPAT5和FBL在蛋白水平上并不存在相关性。K201位点突变使AGPAT5对肝癌细胞迁移的抑制作用减弱,并使AGPAT5与FBL的结合减弱。 结论:敲低 AGPAT5能够显著提高肝癌细胞迁移能力。AGPAT5可以与FBL相互作用,在无血清饥饿刺激下,AGPAT5或通过K201位点的乙酰化加强与FBL的结合,从而更有效地遏制FBL,进而抑制肝癌细胞迁移。但这种抑制作用并非来自AGPAT5的代谢酶活性,而是由非代谢作用所驱动。 [关键词]1-酰基甘油-3-磷酸O-酰基转移酶5;肝癌转移;原纤维蛋白;K201位点乙酰化;非代谢作用 [Abstract] Background and purpose:Metabolic reprogramming occurs during tumor progression, and 1-acylglycerol-3-phosphate O-acyltransferase (AGPAT), as a key enzyme in the de novo synthesis of triacylglycerol (TAG), is closely associated with tumor progression. However, one of the isoforms, AGPAT5, has been studied in cancer in a very limited way, and this study aimed to provide a new perspective on the role of AGPAT5 in hepatocellular carcinoma and its potential molecular mechanisms, providing novel ideas for the diagnosis and treatment strategies of liver cancer. Methods: AGPAT5 was knocked down in a variety of hepatocellular carcinoma cell lines using lentiviral infection, and the effects of AGPAT5 on the functions of hepatocellular carcinoma cell proliferation, migration and resistance to anoikis were detected in vitro by experiments such as Taipan blue counting, scratching, transwell and plate cloning. The wild-type or enzyme activity-deficient form of AGPAT5 was rescued to investigate whether AGPAT5, as a metabolic enzyme, plays a classical role in regulating the migration of hepatocellular carcinoma cells. We constructed a tail vein metastasis model in nude mice to validate the cellular phenotype in vitro from the in vivo level. Immunoprecipitation mass spectrum (IP-MS) identified proteins interacting with AGPAT5 and verified by co-immunoprecipitation (coIP). Protein post-translational modification identification was performed to analyze the potential modification sites of AGPAT5, and in vitroexperiments were performed to explore the effects of the point mutation before and after the point mutation on the migration of hepatocellular carcinoma cells. CoIP was performed to explore the binding of AGPAT5 to the interacting protein before and after the mutation of the site. We determined its role in cell phenotype by knocking down interacting proteins. Rescue experiments were used to verify whether AGPAT5 exerts its effects through the interacting protein. We detected the expression levels of AGPAT5 and the interacting protein in wild-type hepatocellular carcinoma cell lines to examine their correlation. Results:Knockdown of AGPAT5 increased the tolerance to serum-free starvation and promoted hepatocellular carcinoma cell migration, but did not affect proliferation and anoikis. However, deletion of enzyme activity did not affect the inhibition of hepatocellular carcinoma cell migration by AGPAT5. Knockdown of AGPAT5 promoted lung and liver metastasis of hepatocellular carcinoma cells in nude mice. AGPAT5 could interact with fibrillarin (FBL), and the interaction was strengthened under serum starvation conditions. Curbing FBL expression inhibited hepatocellular carcinoma cell migration, and the effect was similar to that of overexpression of AGPAT5. Inhibition of FBL expression weakened the promoting effect of AGPAT5 knockdown on hepatocellular carcinoma cell migration; In the hepatocellular carcinoma cell lines examined, AGPAT5 and FBL did not show any correlation at the protein level. The inhibitory effect of AGPAT5 on hepatocellular carcinoma cell migration was attenuated by the K201 site mutation, and the K201 site mutation attenuated the binding of AGPAT5 to FBL. Conclusion:Knockdown of AGPAT5 can significantly enhance the migratory ability of hepatocellular carcinoma cells. AGPAT5 can interact with FBL, and in the absence of serum starvation stimulation, AGPAT5 strengthen its binding to FBL through acetylation of the K201 site, thereby more effectively inhibiting FBL, consequently inhibiting the migration of hepatocellular carcinoma cells. But this inhibitory effect is not derived from the metabolic enzyme activity of AGPAT5, but driven by non-metabolic function. [Key words]1-acylglycerol-3-phosphate O-acyltransferase 5; Liver cancer metastasis; Fibrillarin; K201 site acetylation; Non-metabolic function 肝癌是全球第6大常见癌症,也是第3大癌症相关死亡原因 [1]。原发性肝癌的两种主要类型是起源于肝细胞的肝细胞癌(hepatocellular carcinoma,HCC)和起源于肝内胆管细胞的肝内胆管癌(intrahepatic cholangiocarcinoma,ICC),其中HCC约占85% [2]。由于世界各国对接种疫苗的重视,肝癌主要的危险因素逐渐从以前公认的乙型肝炎病毒(hepatitis B virus,HBV)、丙型肝炎病毒(hepatitis C virus,HCV)等转变为肥胖、糖尿病和代谢综合征 [3]。随着目前生活水平的提高,生活方式发生了巨大变化,我们需要更关注肥胖、糖尿病和代谢综合征带来的患病风险。另一方面,肝癌患者在手术后5年内复发率超过70%,癌症相关死亡率约为80% [4]。而肝癌患者死亡率居高不下的原因是治疗的不及时和高发的肝内转移 [5-6]。因此,研究肝癌转移背后的分子机制对改善肝癌患者预后至关重要。 溶血磷脂酰基转移酶(lysophospholipid acyltransferases,LPLATs)是一组调节磷脂代谢的关键酶,通过脱酰基-再酰化作用实现磷脂的重塑合成和重构。哺乳动物中的14种LPLATs分为膜结合O-酰基转移酶(membrane-bound O-acyltransferase,MBOAT)家族和1-酰基甘油-3-磷酸O-酰基转移酶(1-acylglycerol-3-phosphate O-acyltransferase,AGPAT)家族 [7-8]。其中,AGPAT家族成员包括AGPAT1-5、溶血磷脂酰胆碱酰基转移酶(lysophosphatidylcholine acyltransferases,LPCATs)家族的大部分成员、甘油-3-磷酸酰基转移酶(Glycerol-3-phosphate acyltransferases,GPATs)和线粒体磷脂-溶血磷脂转酰基酶 [7]。 AGPAT是三酰甘油(triacylglycerol,TAG)从头合成途径第二步的关键酶,可以催化溶血磷脂酸(lysophosphatidic acid,LPA)转化为磷脂酸(Phosphatidic acid,PA)。AGPAT有5种亚型,分别由不同的基因编码,其中AGPAT5与其他家族成员的序列相似性最低 [9]。大量研究 [10-17]表明,AGPAT1、AGPAT2及AGPAT4在多种癌症中高表达与患者预后差相关,但在卵巢癌患者中,AGPAT1表达却下调 [18],提示AGPAT家族在癌症中的作用并不是单一的,而是受到多种复杂机制的调控。研究 [19-20]显示, AGPAT5会抑制结直肠癌细胞增殖和迁移,并促进细胞凋亡,但AGPAT5又可以促进肝母细胞瘤增殖、迁移和侵袭 [21],提示AGPAT5与肿瘤转移既有密切的相关性,又存在强烈的异质性。目前,癌症领域中关于AGPAT5的研究已经取得了一定进展。然而,AGPAT5在调节肿瘤细胞相关表型背后的分子机制,以及相关的信号通路等方面的研究仍存在不足,特别是在肝癌研究领域中。因此,本研究旨在探索AGPAT5与肝癌转移的关系并深入探讨其潜在的分子机制。 1 材料和方法 1.1 主要试剂 胎牛血清(fetal bovine serum,FBS)、DMEM培养基及RPMI-1640培养基均购自美国Corning公司,胰蛋白酶购自美国Thermo Fisher Scientific公司,青-链霉素(双抗)购自苏州新赛美生物科技有限公司,Flag-tag、HA-tag及ACTB等主要抗体购自美国Cell Signaling Technology公司,EZ Trans细胞转染试剂购自和元李记(上海)生物技术有限公司,细胞培养所需耗材购自广州洁特生物过滤股份有限公司。 1.2 细胞培养和转染 人的HCC细胞系SNU449、Huh7、SNU387、SNU475、HCCLM9和MHCC97H购自上海中乔新舟生物科技有限公司。所有细胞均置于含有10%FBS的完全培养基中,在37 ℃、CO 2 体积分数为5%的条件下培养。 1.3 蛋白质印迹法(Western blot) 收集细胞,用RIPA裂解液提取细胞总蛋白,将蛋白样品与上样缓冲液混匀在95 ℃下加热10 min,于10%十二烷基硫酸钠聚丙烯酰胺凝胶电泳(sodium dodecylsulphate polyacrylamide gel electrophoresis,SDS-PAGE)胶中分离,分离的蛋白转移到硝酸纤维素膜上,采用5%脱脂牛奶封闭1 h,将一抗在4 ℃下温育过夜。含有吐温-20三乙醇胺缓冲盐溶液(tris-buffered saline Tween,TBST)清洗3次,每次10 min,将二抗在室温下温育1 h,经增强化学发光(enhanced chemiluminescence,ECL)试剂显影后拍照分析。 1.4 细胞划痕实验 将细胞接种到6孔板中,待细胞贴壁后,使用灭菌的10 μL移液器吸头在细胞层上划痕,磷酸缓冲盐溶液(phosphate-buffered saline,PBS)清洗1~2次,并换成无血清的培养液继续培养。使用显微镜分别获取0、24、48、72和96 h的细胞迁移的图像,直至细胞划痕愈合为止。 1.5 平板克隆实验 将1 000~2 000个细胞均匀地接种到6孔板中,在培养箱中连续培养7 d。用4%多聚甲醛溶液固定后,用0.1%结晶紫溶液染色并拍照分析。 1.6 Transwell实验 提前将完全培养液加进配套的24孔板底部,然后将细胞重悬于无血清培养基中,并接种到transwell小室中。培养一段时间后,取出小室用4%多聚甲醛溶液固定30 min,用0.1%结晶紫溶液染色30 min,并在显微镜下观察。 1.7 免疫共沉淀(co-immunoprecipitation,coIP) 收集细胞,用IP裂解液4 ℃裂解细胞 30 min,4 ℃ 8 000×g离心10 min,去除细胞沉淀。取部分裂解液上清与上样缓冲液混匀,在95 ℃下加热10 min,作为样品的全细胞裂解液(Input)。从每个10 cm的培养皿中取出5 uL 抗Flag琼脂糖珠(鼠源),清洗1~2次以去除储液,然后与裂解液混合,4 ℃温育过夜。第2天 4 ℃ 1 000×g离心10 min,去上清。清洗琼脂糖珠3次,加入一定量的Flag多肽室温温育30 min,取全部上清,与上样缓冲液混匀,在95 ℃下加热10 min,作为IP的样品。所有样品经过Western blot检测。 1.8 构建裸鼠尾静脉注射移植瘤模型 4~6周龄BALB/c裸鼠(雌性)购自广东药康生物科技有限公司。将细胞重悬于PBS中,对裸鼠的尾静脉进行注射。45 d后处死裸鼠并称重。收取裸鼠的肝脏和肺,并进行H-E染色和免疫组织化学(immunohistochemistry,IHC)染色。 1.9 统计学处理 采使用Prism 9.0软件对数据进行统计分析。数据描述以 x±s 表示。两组或两组以上的比较用Student t 检验或ANOVA检验,多组间两两比较采用LSD事后检验。采用双侧检验, P <0.05为差异有统计学意义。 2 结 果 2.1 敲减AGPAT5促进肝癌细胞迁移 利用短发夹RNA(short hairpin RNA,shRNA)对靶基因 AGPAT 5进行了敲减(简称shAGPAT5),以非靶标shRNA(non-target shRNA)作为对照(简称shNT),分别构建肝癌细胞系MHCC97H、SNU387、SNU475、 HCCLM9、SNU449、Huh7的shAGPAT5及shNT细胞株。用上述构建好的细胞株进行transwell实验,发现在SNU387、SNU475、HCCLM9、SNU449、Huh7中敲减 AGPAT 5后都比shNT的迁移能力强( P <0.05,图1),其中,SNU449和Huh7 shAGPAT5组的细胞迁移数更显著高于对照组( P <0.01),所以最终选用SNU449和Huh7作为实验细胞系。 图1 敲减 AGPAT 5促进肝癌细胞迁移 Fig. 1 Knockdown of AGPAT 5 promotes HCC cells migration Transwell migration assays were assessed in AGPAT5-knockdown HCC cell lines. Representative images (scale bar: 100 μm) and statistical analyses of the migrated cells are shown. 2.2 敲减 AGPAT 5促使肝癌细胞耐受无血清饥饿 为进一步挖掘AGPAT5影响HCC细胞迁移的原因,上述构建的SNU449、Huh7的shAGPAT5及shNT稳定株在无血清饥饿刺激下通过锥虫蓝计数检测其细胞活力,结果显示,SNU449和Huh7敲减 AGPAT 5后,都更显著耐受无血清饥饿( P <0.05,图2A)。然而,在正常培养条件下进行锥虫蓝计数检测其细胞增殖能力时,发现敲减 AGPAT 5后对肝癌细胞增殖无显著影响( P >0.05,图2B)。平板克隆实验结果进一步表 明,敲减 AGPAT 5也未显著影响肝癌细胞的抗失巢凋亡能力( P >0.05,图2C、2D)。上述结果提示敲减 AGPAT 5或通过耐受无血清饥饿而导致肝癌细胞迁移水平升高。 图2 敲减 AGPAT 5促使肝癌细胞耐受无血清饥饿 Fig. 2 Depletion of AGPAT 5 induces tolerance to serum-free starvation in HCC cells A, B: SNU449 and Huh7 cells stably expressing shNT or shAGPAT5 were treated with or without FBS starvation in the indicated times, and then, cell number was assessed. C: Colony formation assays were performed in AGPAT5-depleted SNU449 and Huh7 cells. Representative images and statistical analyses of the colony cells are shown. 2.3 AGPAT5酶活性缺失并不影响肝癌细胞迁移能力 代谢重编程在肿瘤的发生、发展过程中发挥重要作用,AGPAT5作为TAG从头合成途径关键酶的其中一种亚型,是否通过代谢作用调控肝癌转移成为本研究的重点。为探究AGPAT5的酶活性对肝癌细胞迁移的影响,构建SNU449、 Huh7的shAGPAT5及shNT稳定株,分别回补AGPAT5野生型(rescue AGPAT5 wild type,rAGPAT5-WT)或其酶活性缺失的类型(rescue AGPAT5 enzyme deficiency,rAGPAT5-ED)或对照(empty vector,EV)(图3A)。通过transwell实验验证,AGPAT5酶活性缺失与否都抑制了肝癌细胞迁移,且两者的抑制水平差异不显著( P >0.05,图3B、3C)。上述结果提示AGPAT5并不是通过经典的代谢作用来抑制肝癌细胞迁移。 图3 AGPAT5失活并不影响肝癌细胞迁移能力 Fig. 3 AGPAT5 inactivation does not affect the migration of HCC cells A: Construct the enzyme deficiency plasmid. B, C: Transwell migration assays were performed in AGPAT5-depleted SNU449 and Huh7 cells rescued with EV or rAGPAT5-WT or rAGPAT5-ED. Representative images (scale bar: 100 μm) and statistical analyses of the migrated cells are shown. 2.4 敲减 AGPAT 5促使肝癌细胞在小鼠体内进行转移 为充分模拟敲减 AGPAT 5的肝癌细胞在体内中的转移情况,构建SNU449的shAGPAT5及shNT稳定株,并建立裸鼠尾静脉转移模型,每组各6只。45 d后处死裸鼠并称重(图4A),shAGPAT5组裸鼠的体重显著轻于shNT组( P <0.01,图4B),分别收集对应的肝脏和肺样本(图4C)。包埋切片后通过H-E染色和Ki-67免疫染色比较分析肝癌细胞在裸鼠体内的转移情况,结果显示,敲减 AGPAT 5显著促进了肝癌细胞在裸鼠体内的肝脏和肺转移( P <0.01,图4D~4F)。综合上述结果,AGPAT5对肝癌转移的抑制作用在体内外皆得到了验证。 图4 敲减 AGPAT 5促使肝癌细胞在小鼠体内进行转移 Fig. 4 Depletion of AGPAT 5 promotes the metastasis of HCC cells in mice A, B, C: SNU449-shNT cells or SNU449-shAGPAT5 cells were implanted into randomized athymic nude mice via a tail vein injection (6 mice per group). Then, after 45 d of inoculation, the representative images of these implanted mice was carried out, the body weight of mice was quantified. The representative images of lung and liver metastasis are presented. D, E, F: Representative images of liver and lung tissues dissected 45 d after the inoculation and H-E and Ki-67 stained metastatic nodules are presented (scale bar: 50 μm) and statistical analyses of metastatic nodules are shown. 2.5 AGPAT5可能通过遏制原纤维蛋白(fibrillarin,FBL)来抑制肝癌细胞迁移 癌症的发生、发展通常与蛋白质之间的相互作用异常相关,为深入了解AGPAT5在癌症转移中的作用机制,进一步挖掘与AGPAT5相互作用的蛋白质。构建SNU449 Flag-AGPAT5过表达稳定株,有或无血清处理后进行免疫沉淀质谱联用(immunoprecipitation mass spectrum,IP-MS)鉴定(图5A)。结果显示,AGPAT5在无血清饥饿刺激下与FBL的结合增加,在SNU449和Huh7细胞系中都进行了coIP验证(图5B)。为进一步确认AGPAT5结合FBL对肝癌细胞迁移的影响,构建SNU449、Huh7敲减FBL和shNT的稳定株。通过transwell实验发现,遏制FBL与过表达AGPAT5都显著抑制了肝癌细胞迁移( P <0.05,图5C、5D)。通过共转染shAGPAT5和shFBL进行回复实验,以进一步验证AGPAT5是否通过调节FBL来促进细胞迁移(图5E)。结果显示,抑制FBL显著减弱了敲低AGPAT5对SNU449和Huh7细胞迁移的促进作用( P <0.01,图5F)。另外,通过收集野生型肝癌细胞裂解液分别检测AGPAT5和FBL的表达水平(图5G),发现两者并无显著相关性( P >0.05,图5H)。综合上述结果推测,在无血清饥饿刺激下AGPAT5可能通过加强与FBL结合遏制了FBL,从而达到抑制肝癌细胞迁移的效果。 图5 AGPAT5可能通过遏制FBL来抑制肝癌细胞迁移 Fig. 5 AGPAT5 may inhibit the migration of HCC cells by suppressing FBL A: Mass spectrometry analyses of AGPAT5-associated proteins were performed in SNU449 cells stably expressing FLAG-AGPAT5. The precursor ion was fragmented by collision-induced dissociation and analyzed in an ion trap. The database search engine (Andromeda) score was matched to the identified peptide. B: SNU449 and Huh7 cells were treated with or without FBS for 1 h. C, D: Transwell migration assays were performed in AGPAT5-depleted or AGPAT5-overexpressed or FBL-depleted SNU449 and Huh7 cells. Representative images (scale bar: 100 μm) and statistical analyses of the migrated cells are shown. E, F: Transwell assays revealed that FBL depletion abrogated the promoted migration caused by AGPAT5 knockdown in SNU449 and Huh7 cells. G, H: HCC cell lysate to test the relative expression level of AGPAT5 and FBL. IB: immunoblot; IP: immunoprecipitation. 2.6 AGPAT5的K201是负责抑制FBL的关键位点 为进一步探索AGPAT5是否通过自身修饰而影响肝癌细胞迁移,通过蛋白质翻译后修饰鉴定分析得到AGPAT5潜在的4个修饰位点,分别是Y177、T182、S186位点的磷酸化和K201位点的乙酰化。在SNU449中分别构建上述位点突变及EV稳定株,通过transwell实验探究这些位点突变对AGPAT5抑制肝癌细胞迁移的影响(图6A),发现T182、K201位点的突变使AGPAT5对细胞迁移的抑制效果减弱,但K201位点更加显著( P <0.05,图6B),而其余突变位点并无显著变化( P >0.05,图6B)。划痕实验结果进一步印证,K201位点突变后,AGPAT5对肝癌细胞迁移的抑制受到显著影响( P <0.05,图6C、6D)。由此猜想,K201可能是影响AGPAT5与其相互作用蛋白FBL结合的关键位点,并在有或无血清条件下进行coIP验证(图6E)。结果显示,在无血清饥饿刺激下,K201位点突变后使AGPAT5与FBL的结合减弱。综合上述结果,AGPAT5的K201乙酰化可能是抑制FBL的关键位点,从而达到抑制肝癌细胞迁移的效果。 图6 AGPAT5的K201是负责抑制FBL的关键位点 Fig. 6 K201 of AGPAT5 is the key site for FBL repression A, B, C, D: Transwell migration assays and wound healing assays of SNU449-AGPAT5 (WT), SNU449-AGPAT5 (Y177A), SNU449-AGPAT5 (T182A), SNU449-AGPAT5 (S186A) and SNU449-AGPAT5 (K201A) cells. Representative images (A) (scale bar: 100 μm) and statistical analyses of the migrated cells are shown. Representative images (C) (scale bar: 2.5 mm) and statistical analyses of gap distance are shown. E: SNU449 cells were treated with or without FBS for 1 h. 3 讨 论 在肿瘤的发生、发展过程中往往伴随着代谢重编程,而脂质代谢作为细胞代谢的重要组成部分,已成为癌症诊断、监测和治疗的焦点 [22]。越来越多的研究 [23]表明,脂质代谢与肿瘤的发生、发展及患者预后密切相关。而磷脂作为脂质的重要组成成分,既是生物膜的组成成分,又可以参与细胞的信号转导等。哺乳动物体内的磷脂经肯尼迪途径新生合成,其中大部分新生合成的磷脂还需要经历磷脂重塑,这也是膜中磷脂结构多样性的重要原因 [24]。 AGPAT是生物通过甘油-3-磷酸途径生物合成磷脂和TAG的关键酶,但在癌症领域中对于AGPAT5的研究较少。既往研究 [19-20]表明, AGPAT5会抑制结直肠癌细胞增殖和迁移,并促进其细胞凋亡。但同时AGPAT5又可以促进肝母细胞瘤增殖、迁移和侵袭 [21],提示AGPAT5与肿瘤转移密切相关,但发挥的作用却不尽相同。目前对于AGPAT5介导的表型背后的分子机制及与其他信号通路之间的联系仍缺乏深入理解。而肝脏作为脂质代谢的主要器官,其脂质代谢重编程是肝癌细胞应对不利环境的一种适应性改变。因此,AGPAT5在肝癌,尤其是其主要类型HCC中的研究尤为重要。 肝癌的高复发率和转移率是患者预后差的主要因素 [5],因此防治肝癌转移是肝癌治疗的关键。本研究结果表明,AGPAT5可以使肝癌细胞对血清饥饿敏感并抑制肝癌细胞迁移,并在裸鼠体内构建尾静脉转移模型中得到进一步验证。本研究探索了AGPAT5作为代谢酶是否通过代谢途径对肝癌细胞迁移进行调控,结果显示, AGPAT5对肝癌转移的抑制并非源自其经典的代谢功能。越来越多的研究 [25-26]表明,代谢酶不仅通过其传统的代谢作用调控肿瘤的发生、发展,还通过其他非代谢途径实现这种调控。 癌症的发生、发展通常与蛋白质之间的相互作用异常相关,为深入了解AGPAT5在癌症转移中的作用机制,利用IP-MS挖掘出与AGPAT5潜在的相互作用蛋白可能是FBL。FBL作为必需的核仁蛋白,主要负责核糖体RNA和蛋白质的甲基化,在核糖体生物合成和蛋白质合成中发挥重要作用。有研究 [27-29]显示,FBL在肝癌、肺癌、乳腺癌和结直肠癌等多种癌症中高表达,FBL不仅可以增强肿瘤细胞增殖能力,还与化疗耐药性、代谢重编程相关。例如,FBL在肿瘤细胞遭受顺铂等化疗药物导致的DNA损伤时,能够促进DNA损伤的修复 [27-28]。不仅如此,高表达FBL的肝癌患者通常预后较差。体外实验 [30]结果显示,敲除FBL可以显著抑制HCC细胞增殖、干性、迁移和侵袭,这与本研究中敲低FBL后显著抑制迁移的结果一致。FBL还在肿瘤的发生、发展中扮演着重要角色。一方面,FBL参与核糖体RNA的加工,并通过调控翻译影响肿瘤细胞的生长;另一方面,FBL作为转录共调节因子,能够增强下游靶基因的表达,促进细胞生长或DNA损伤修复。本研究发现FBL与AGPAT5之间存在相互作用,为探索这两个基因在肝癌中的作用提供了新思 路。 蛋白质的翻译后修饰可以调节其活性、稳定性、相互作用和细胞信号转导等,这些修饰通过改变蛋白质的化学性质或空间构象,从而影响其生物学功能和相应的细胞过程。本研究发现,AGPAT5与FBL的相互作用在无血清饥饿刺激下得到了加强,结合FBL的关键位点可能是AGPAT5的K201乙酰化。 综上所述,本研究通过一系列生物学实验鉴定了肝癌中一个较新的抑癌型代谢酶AGPAT5。该代谢酶在肝癌的转移过程中发挥重要作用,抑制AGPAT5可以显著促进肿瘤转移,因此AGPAT5有望成为针对恶性肿瘤的潜在分子诊断标志物和治疗靶点。 利益冲突声明:所有作者均声明不存在利益冲突。 作者贡献声明: 陈奕君:完成实验,撰写并修改文章; 刘雨航:参与实验,修改文章; 段海波:参与实验,修改文章; 王雄军:指导实验,审核并修改文章。 [参考文献]
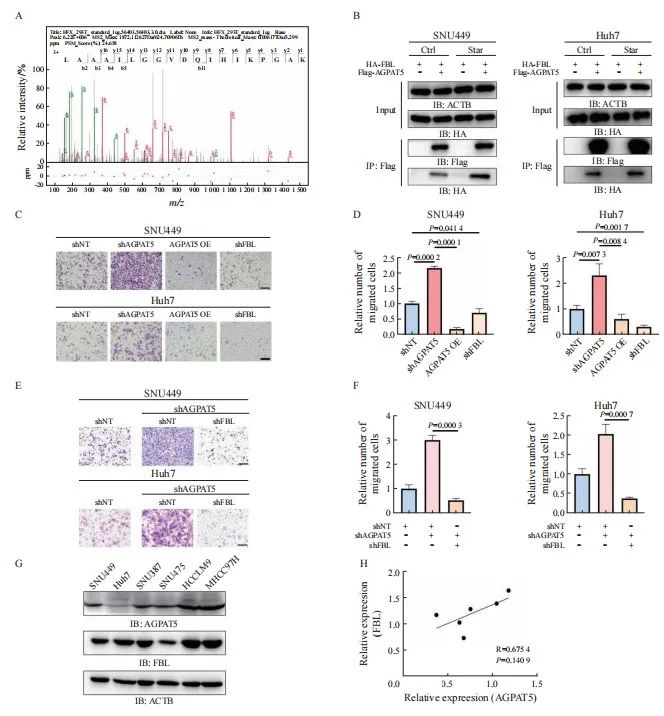
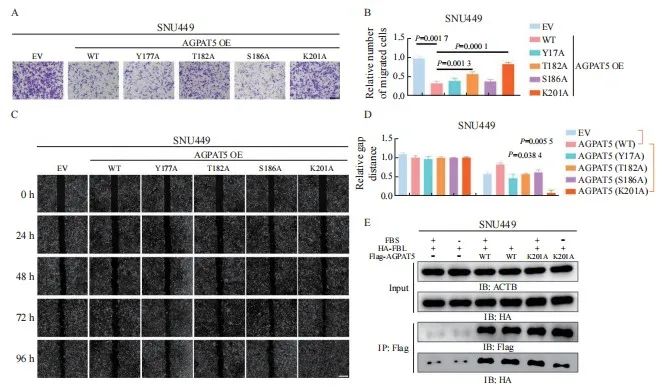
本文责任编辑:李广涛
链接:http://www.lewenyixue.com/2024/10/31/%E3%80%90%E8%AE%BA%E8%91%97%E3%80%91%7C%20AGPAT5%E5%9C%A8%E8%82%9D%E7%99%8C%E4%B8%AD%E7%9A%84%E5%8A%9F%E8%83%BD%E4%B8%8E/


