Background: Incorporating brentuximab vedotin into the treatment of advanced-stage classic Hodgkin's lymphoma improves outcomes in adult and pediatric patients. However, brentuximab vedotin increases the toxic effects of treatment in adults, more than half of pediatric patients who receive the drug undergo consolidative radiation, and relapse remains a challenge. Programmed death 1 blockade is effective in Hodgkin's lymphoma, including in preliminary studies involving previously untreated patients.
Methods: We conducted a phase 3, multicenter, open-label, randomized trial involving patients at least 12 years of age with stage III or IV newly diagnosed Hodgkin's lymphoma. Patients were randomly assigned to receive brentuximab vedotin with doxorubicin, vinblastine, and dacarbazine (BV+AVD) or nivolumab with doxorubicin, vinblastine, and dacarbazine (N+AVD). Prespecified patients could receive radiation therapy directed to residual metabolically active lesions. The primary end point was progression-free survival, defined as the time from randomization to the first observation of progressive disease or death from any cause.
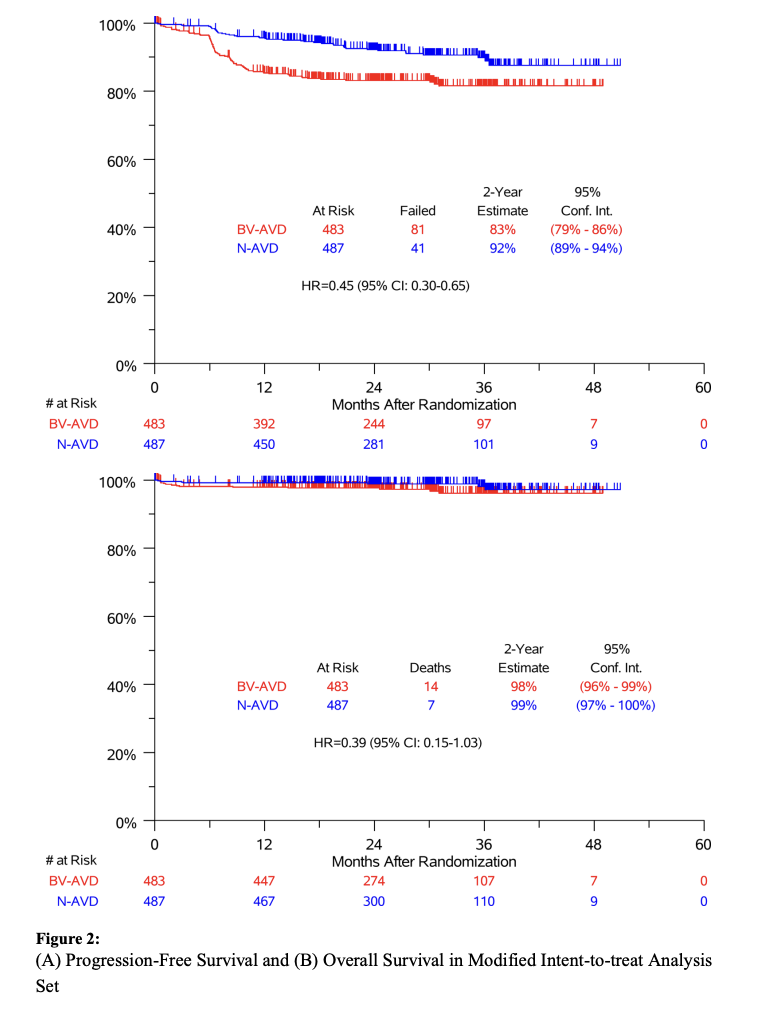
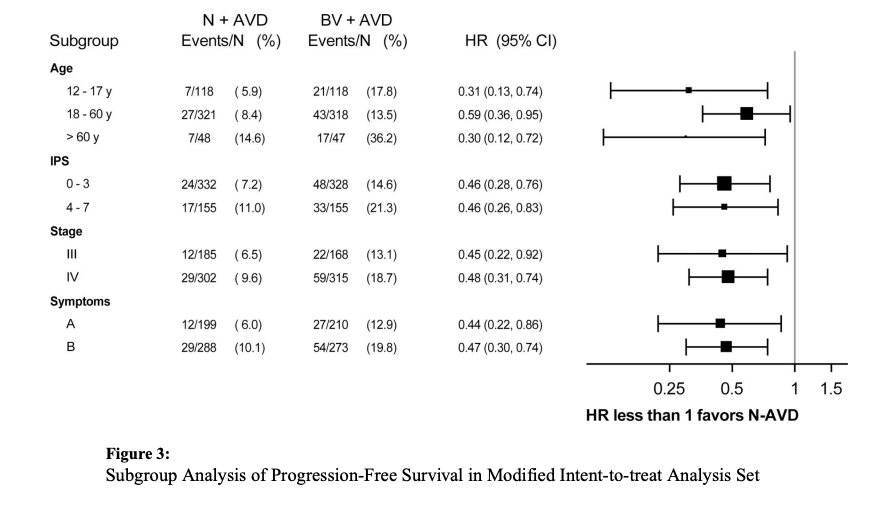
Results: Of 994 patients who underwent randomization, 970 were included in the intention-to-treat population for efficacy analyses. At the second planned interim analysis, with a median follow-up of 12.1 months, the threshold for efficacy was crossed, indicating that N+AVD significantly improved progression-free survival as compared with BV+AVD (hazard ratio for disease progression or death, 0.48; 99% confidence interval [CI], 0.27 to 0.87; two-sided P = 0.001). Owing to the short follow-up time, we repeated the analysis with longer follow-up; with a median follow-up of 2.1 years (range, 0 to 4.2 years), the 2-year progression-free survival was 92% (95% CI, 89 to 94) with N+AVD, as compared with 83% (95% CI, 79 to 86) with BV+AVD (hazard ratio for disease progression or death, 0.45; 95% CI, 0.30 to 0.65). Overall, 7 patients received radiation therapy. Immune-related adverse events were infrequent with nivolumab; brentuximab vedotin was associated with more treatment discontinuation.
Conclusions: N+AVD resulted in longer progression-free survival than BV+AVD in adolescents and adults with stage III or IV advanced-stage classic Hodgkin's lymphoma and had a better side-effect profile. (Funded by the National Cancer Institute of the National Institutes of Health and others; S1826 ClinicalTrials.gov number, NCT03907488 .).